- Caused by missing immunoglobins (antibodies)
- Antibodies control the immune system and prevent illness
- Patients are chronically ill from severe, persistent, recurrent infections
made from plasma
Plasma-derived medicines are often the only therapies for many rare and chronic diseases. Given that plasma cannot be produced artificially, its lifesaving proteins can only be obtained from eligible human donors.
Plasma cannot be made in a laboratory
Human plasma is the unique and indispensable starting material for the manufacturing of plasma-derived medicinal products.
These therapies must be tested and approved
Plasma collection adheres to safety protocols to protect the health of donors and patients.
EVERYDAY MEDICINES
Plasma-derived medicines are often the only therapies for many rare and chronic diseases. Given that plasma cannot be produced artificially, its lifesaving proteins can only be obtained from eligible human donors.
Burn patients
Severe burns can lead to life-threatening complications, including fluid loss and infection. Plasma-derived medicines, such as albumin, can help replenish lost fluids in burn patients. Additionally, fibrin sealants derived from plasma can help in wound healing and reduce the risk of infections.
Cancer care
Plasma-derived medicines often play a supportive role in the treatment of cancer patients. Plasma-derived immunoglobulins (IG) can be used to address treatment-related immunosuppression and reduce the risk of infections in patients undergoing chemotherapy or radiation. Additionally, plasma-derived clotting factors can help cancer patients who experience clotting disorders or increased risk of bleeding due to their cancer or related treatment.
In addition, albumin is also used as a carrying agent for some cancer medications, due to it being preferentially internalized by tumor cells. This can prolong the half-life of otherwise rapidly cleared drugs and promote their accumulation within tumors.
Hepatitis B
A plasma-derived medicine with hepatitis B antibodies, called Anti-HBV IG, can help protect people from hepatitis B virus (HBV). Anti-HBV is used as a postexposure preventative measure in individuals who are at risk of acquiring HBV, including health care workers and first responders treating a patient with HBV, as well as babies born to mothers with the virus.
Liver conditions
Plasma-derived medicines such as albumin and prothrombin complex concentrates can be beneficial in managing complications associated with liver conditions. Albumin infusions help maintain fluid balance, stabilize blood pressure, and reduce the risk of abnormal swelling and fluid buildup in patients with cirrhosis.
Some liver disease patients experience an impairment in their own body’s ability to produce clotting factors and can require fresh frozen plasma or plasma-derived clotting factors to manage and prevent bleeding.
Organ transplantation
Transplant recipients are at high risk of graft rejection, which occurs when the recipient's immune system attacks and begins destroying the transplanted tissue or organ. To prevent this, recipients are often given immunosuppressive medications, leaving them vulnerable to opportunistic infection. Plasma-derived immunoglobulins can bolster the immune system and minimize the risk of infections, making them an important adjunct in post-transplant care.
Pediatric HIV
Children infected with HIV often experience immune system deficiencies that predispose them to infections. Plasma-derived immunoglobulins help provide passive immunity and boost these patients’ ability to fight infections.
Rh Incompatibility
During pregnancy, a mismatch in the type of rhesus (Rh) factor (a protein found on the surface of red blood cells), between a mother and fetus can lead to hemolytic disease of the newborn (HDN). Rh incompatibility occurs when a Rh-negative mother is carrying a Rh-positive fetus. In that case, the mother’s immune system sees the red blood cells of the fetus as foreign and develops antibodies which attack them.
This can cause severe anemia in the fetus or newborn, as well as jaundice as the broken-down red blood cells produce bilirubin. The level of bilirubin in the infant's blood may range from mild to dangerously high. Plasma-derived Rh immunoglobulins (RhIg) administered during pregnancy can neutralize the fetus’s Rh-positive red blood cells, preventing maternal sensitization and HDN.
Shock and trauma
In cases of shock and trauma, the rapid loss of blood can result in acute clotting disorders. Plasma-derived clotting factors like Factor IX may control bleeding and restore normal clotting function. Administering these factors to trauma patients can be effective in preventing excessive blood loss.
Tetanus
The presence of antibodies and immunoglobulins in plasma equips it to neutralize harmful substances and infectious agents. In cases where individuals are bitten by animals or sustain deep cuts, there is a risk of tetanus infection, which can be life-threatening. Anti-tetanus immunoglobulin provides antibodies that protect against this infection.
Patients treated with plasma-derived medicines
Plasma-derived medicines are relied upon by patients suffering from life-threatening, rare diseases that require regular treatments. Any decline in plasma donations implies serious health consequences for the hundreds of thousands of individuals living with these conditions, as well as countless others facing trauma and emergency medical needs every day.
DISEASES
- Caused by missing immunoglobulins (antibodies)
- Antibodies control the immune system and prevent illness
- Patients are chronically ill from severe, persistent, recurrent infections
- Caused by missing clotting factor protein
- Clotting factors control bleeding
- Patients cannot regulate bleeding
- Can be fatal if bleeding occurs in brain or vital organs
- Cause not certain; immune system attacks nerve coating
- Messages from the brain aren’t delivered to the body if nerve coating is damaged
- Patients experience progressive weakness, loss of limb function, and disability
- Caused by missing Alpha-1 Proteinase Inhibitor
- Alpha-1 Proteinase Inhibitor protects the lungs
- Patients have chronic emphysema and liver damage
- Caused by missing C1 esterase inhibitor protein (C1-INH)
- C1-INH helps regulate inflammation
- Patients have edema (severe swelling)
- Can be fatal if airway obstructed
- Caused by missing clotting factor protein
- Clotting factors control bleeding
- Patients cannot regulate bleeding
- Can be fatal if bleeding occurs in brain or vital organs
SAFETY FIRST
Plasma-derived medicines require constant vigilance for safe products. There are three types of safeguard measures used in plasma donation and manufacturing to ensure safe plasma-derived medicines:

Selection of Starting Material
The donor will check in at reception and complete a health history questionnaire.

Testing for Pathogens
After the plasma is collected, it is tested for suitability for further manufacture,
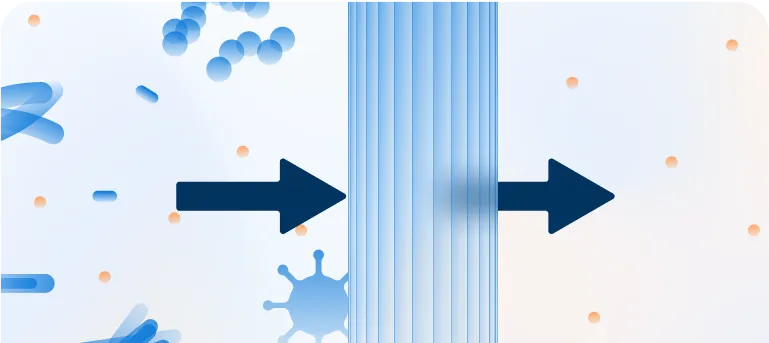
Elimination of Pathogens
Plasma protein fractions are purified to remove unwanted proteins and cleared of potential viruses.
SAFETY PROTOCOLS
Evolving protocols
Unlike traditional pharmaceuticals or other biologics where standard quality assurance practices are sufficient, plasma-derived medicines' safety protocols are constantly evolving due to new and emerging pathogens.
Companies must continuously perform tests to demonstrate that their viral inactivation and removal steps work on new pathogens. For example, through the years companies invested significant time and resources into researching coronaviruses to ensure they do not threaten the safety of plasma-derived medicines.
Current manufacturing protocols are extremely effective against pathogens
The industry has a record of safety from pathogens for more than 30 years.


COST ATTRIBUTED TO MANUFACTURING & RAW MATERIAL*
Plasma-derived medicines take up to 12 months to manufacture. Companies must adhere to rigorous regulatory requirements to ensure manufacturing consistency and pathogen safety. These medications are also 57% more expensive to manufacture than other synthetic medicines.
PHARMACEUTICALS

Compound
mixing

Capsule filling/
tableting

Packaging and
distribution
14% of cost attributed to manufacture & raw materials
PLASMA-DERIVED THERAPIES
Plasma
Collection

Testing

Fractionation

Purification

Filling

Packaging and distribution
Administration to patients
68% of cost attributed to manufacture & raw materials
NON-interchangeable & Unique
One-size-fits-all policies are unsuitable for plasma-derived medicines and endanger patient health. Each therapy is unique due to the pharmacologic and manufacturing differences that exist across different brands and patients’ unique response to the treatments.
Plasma-derived medicines are non-interchangeable, sole-source biologics, therefore it is essential that patients have access to their medically justifiable therapy.
Expert clinical guidelines on non-interchangeability

“It is unacceptable to limit availability of augmentation therapy in any way and especially to a single product.”
Alpha-1 Foundation Medical and Scientific Advisory Committee Clinical Practice Guidelines

“HAE management plans must be individualized to each patient’s needs due to wide variability in HAE symptoms, response to and tolerance of various HAE medications, and numerous factors impacting clinical course and quality of life. Treatment plans should be monitored regularly and adjusted based on the needs of the patient.”
US HAEA Medical Advisory Board 2020 Guidelines for the Management of Hereditary Angioedema

“Given the variable nature of these diseases, individualized treatments depending on patient need and physician judgment are important.”
American Academy of Neurology Therapeutics & Technology Assessment Subcommittee Evidence-based Guidelines



















