Constant Vigilance for Safe Products
Plasma protein therapies require constant vigilance for safe products. There are three types of safeguard measures used in plasma donation and manufacturing to ensure safe plasma protein therapies:
Voluntary industry standards often exceed regulatory requirements.


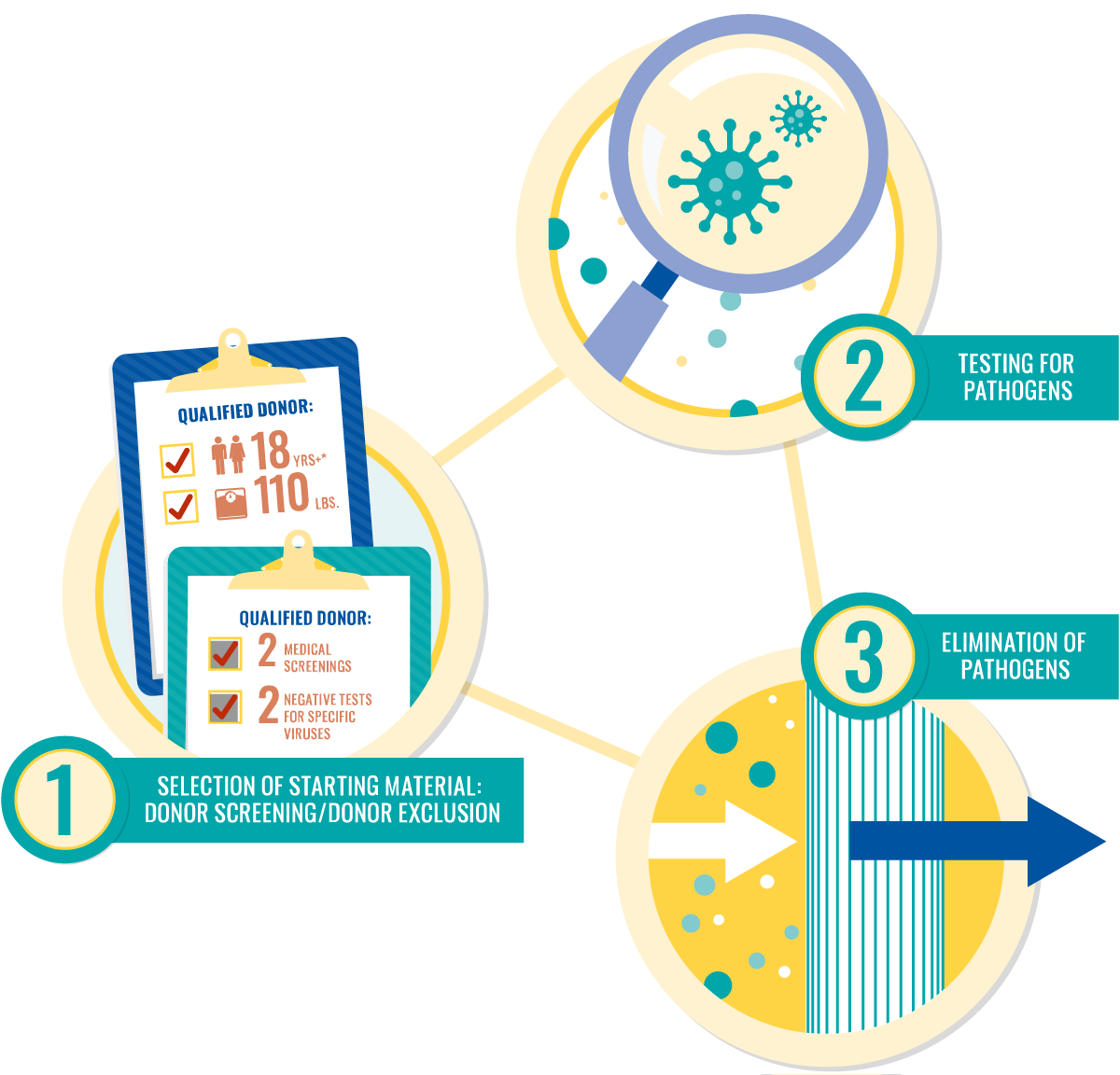
Current manufacturing protocols are extremely effective against pathogens.
The industry has a record of safety from pathogens for more than 30 years.

Evolving Protocols
Unlike traditional pharmaceuticals or other biologics where standard quality assurance practices are sufficient, plasma protein therapies’ safety protocols are constantly evolving due to new and emerging pathogens.
Companies must continuously perform tests to demonstrate that their viral inactivation and removal steps work on new pathogens. For example, through the years companies invested significant time and resources into researching coronaviruses to ensure they do not threaten the safety of plasma protein therapies.


